 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!  Offering SPR-BLI Services - Proteins provided for free!
Offering SPR-BLI Services - Proteins provided for free!
 Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!  Here come GMP Grade Cytokines!Free Sample is available!
Here come GMP Grade Cytokines!Free Sample is available!
Immobilized Hendra virus Pre-Fusion glycoprotein (A263T), His Tag (Cat. No. FUN-H52H4) at 1 μg/mL (100 μL/well) can bind Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (1A7) (Cat. No. FUN-MY2097) with a linear range of 0.6-9.8 ng/mL (QC tested).
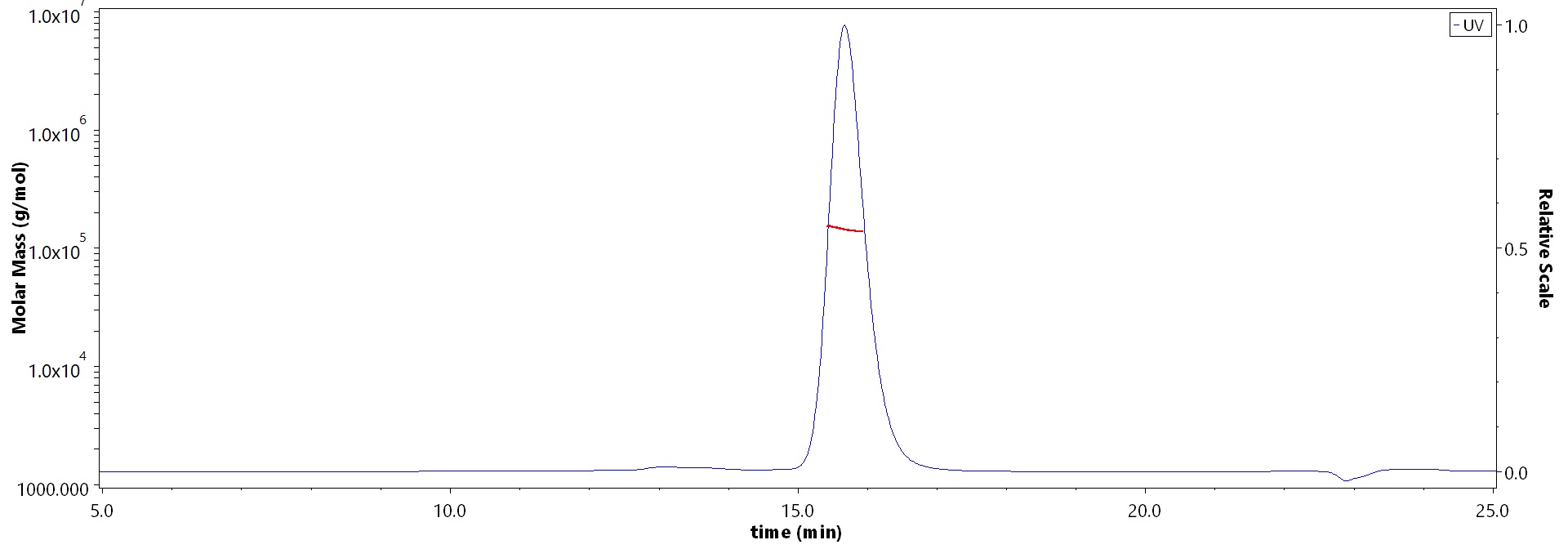
The purity of Monoclonal Anti-Hendra virus F glycoprotein Antibody, Human IgG1 (3F4) (Cat. No. FUN-MY2098) is more than 90% and the molecular weight of this protein is around 135-160 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Nirsevimab | SP-0232; MEDI-8897; Anti-RSV MAb-YTE; Anti-RSV-mAb-D25 | Approved | Aimm Therapeutics, Sanofi, MedImmune Inc | Beyfortus | EU | Respiratory Syncytial Virus Infections | Astrazeneca Ab | 2022-10-31 | Lower Respiratory Tract Infections; Respiratory Syncytial Virus Infections | Details |
| Palivizumab | ABT-315; MEDI-493 | Approved | Abbvie Inc, Medimmune Llc | Synagis | United States | Respiratory Syncytial Virus Infections | Swedish Orphan Biovitrum Ab (Publ) | 1998-06-19 | Bronchopulmonary Dysplasia; Respiratory Syncytial Virus Infections; Infant, Premature, Diseases; Heart Defects, Congenital; Premature Birth | Details |
| Respiratory syncytial virus immune globulin | RSV-IGIV | Approved | Medimmune | RespiGam | United States | Respiratory Syncytial Virus Infections | null | 1996-01-01 | Respiratory Syncytial Virus Infections | Details |
| Nirsevimab | SP-0232; MEDI-8897; Anti-RSV MAb-YTE; Anti-RSV-mAb-D25 | Approved | Aimm Therapeutics, Sanofi, MedImmune Inc | Beyfortus | EU | Respiratory Syncytial Virus Infections | Astrazeneca Ab | 2022-10-31 | Lower Respiratory Tract Infections; Respiratory Syncytial Virus Infections | Details |
| Palivizumab | ABT-315; MEDI-493 | Approved | Abbvie Inc, Medimmune Llc | Synagis | United States | Respiratory Syncytial Virus Infections | Swedish Orphan Biovitrum Ab (Publ) | 1998-06-19 | Bronchopulmonary Dysplasia; Respiratory Syncytial Virus Infections; Infant, Premature, Diseases; Heart Defects, Congenital; Premature Birth | Details |
| Respiratory syncytial virus immune globulin | RSV-IGIV | Approved | Medimmune | RespiGam | United States | Respiratory Syncytial Virus Infections | null | 1996-01-01 | Respiratory Syncytial Virus Infections | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| CPI-RSV-F Vaccine | BLB-201 | Phase 2 Clinical | Blue Lake Biotechnology Inc | Respiratory Syncytial Virus Infections | Details |
| GR-2102 | GR2102; GR-2102 | Phase 1 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd, Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd | Respiratory Syncytial Virus Infections | Details |
| Palivizumab biosimilar (mAbxience) | Phase 1 Clinical | Mabxience Sa | Details | ||
| CPI-RSV-F Vaccine | BLB-201 | Phase 2 Clinical | Blue Lake Biotechnology Inc | Respiratory Syncytial Virus Infections | Details |
| GR-2102 | GR2102; GR-2102 | Phase 1 Clinical | Genrix (Shanghai) Biopharmaceutical Co Ltd, Chongqing Zhixiang Jintai Biopharmaceutical Co Ltd | Respiratory Syncytial Virus Infections | Details |
| Palivizumab biosimilar (mAbxience) | Phase 1 Clinical | Mabxience Sa | Details |
This web search service is supported by Google Inc.
